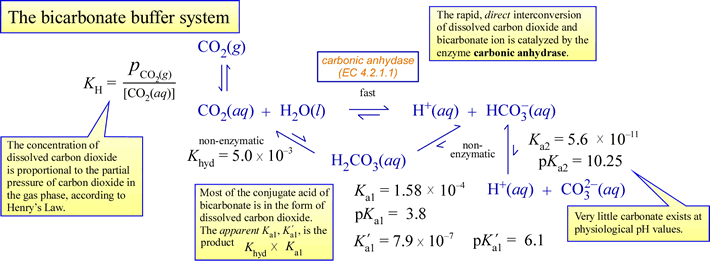
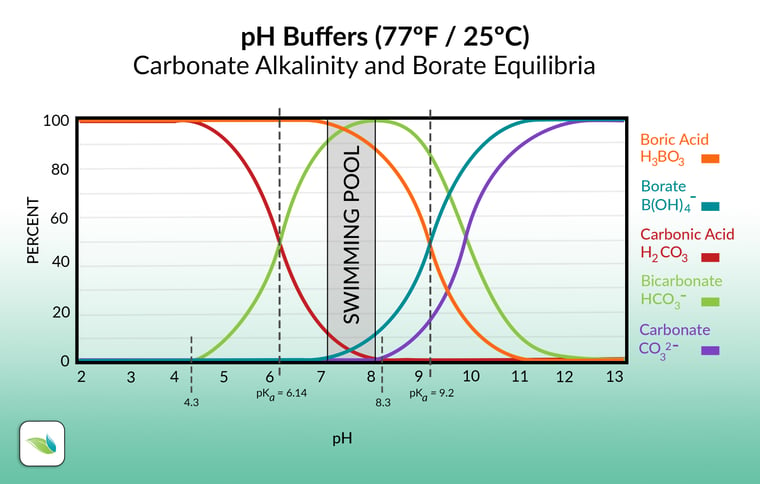
Table 2 from Calculation of the buffering capacity of bicarbonate in the rumen and in vitro. | Semantic Scholar
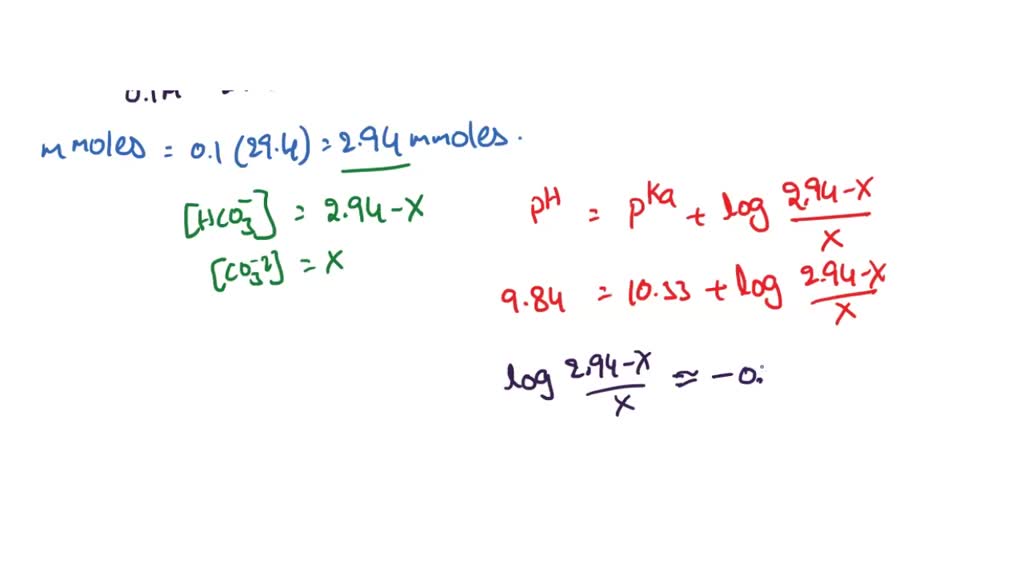
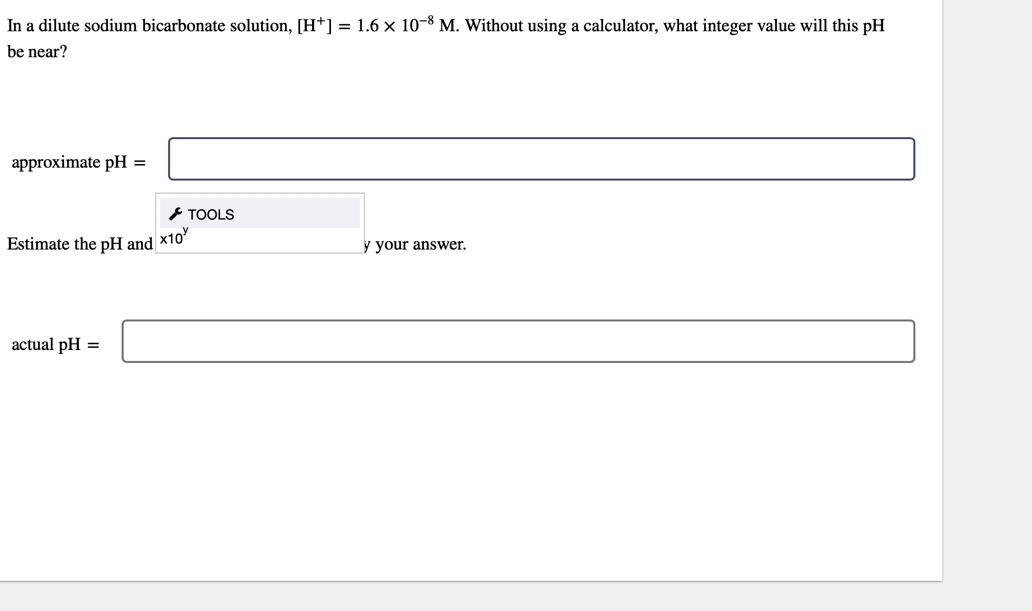
SOLVED: The pKa for the acid dissociation reaction of bicarbonate is 10.33. HCO3-(aq) + H2O(l) ⇄ CO32-(aq) + H3O+(aq) Imagine that your goal is to prepare a buffer with pH 9.84 using

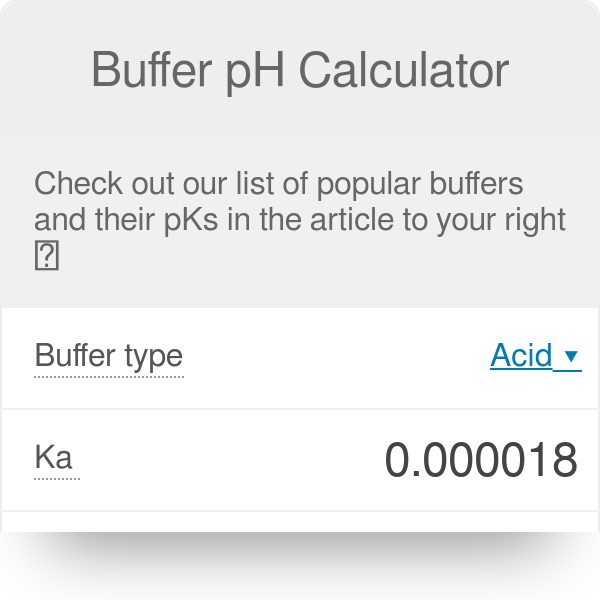
![PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar PDF] A sodium carbonate-bicarbonate buffer for alkaline phosphatases. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/aaa98c1f9de4c398b6a057bf4936ea76736034c7/1-Table1-1.png)